Yeast-mycorrhizae interaction as a strategy to improve tomato production
DOI:
Palavras-chave:
Funneliformis mosseae, Candida saitoana, Saccharomyces eubayanus, Tausonia pullulans, sustainable agricultureResumo
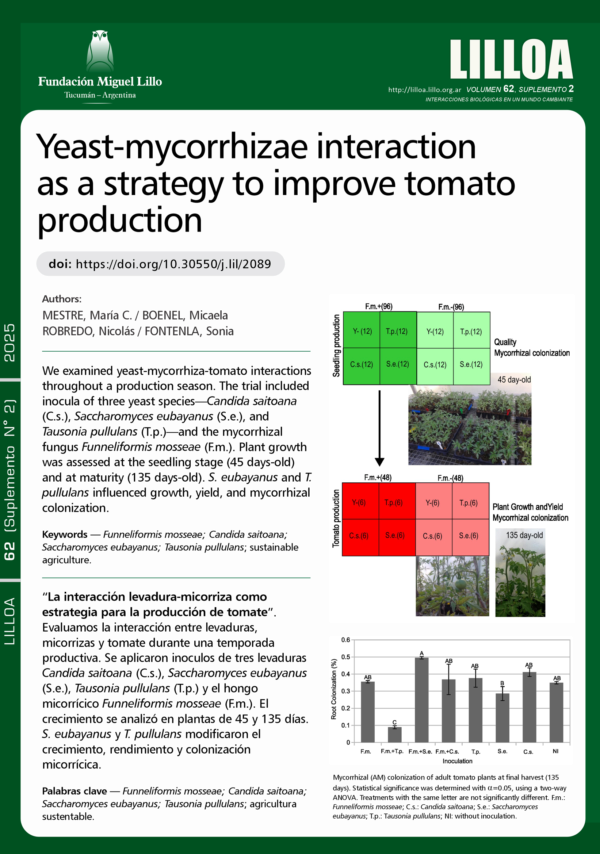
Inoculation with plant growth-promoting microorganisms such as bacteria, mycorrhizal fungi, and soil yeasts may play a promising role in sustainable plant production. This study evaluated the potential of Patagonian yeasts and mycorrhizal fungi to enhance the growth and productivity of tomato plants (Lycopersicum esculentum var. platense) during the production season in Patagonia. A greenhouse experiment was conducted, where plants were inoculated with the mycorrhizal fungus Funneliformis mosseae and the yeasts Candida saitoana, Saccharomyces eubayanus, or Tausonia pullulans. None of the 45-day-old seedlings exhibited mycorrhizal colonization, although F. mosseae inoculation significantly influenced seedling growth. By the end of the production season (135-day-old plants), all plants showed mycorrhizal colonization, and those inoculated with F. mosseae demonstrated increased plant growth and yield. Inoculation with S. eubayanus enhanced both plant yield and mycorrhizal colonization. Conversely, co-inoculation with T. pullulans and F. mosseae was detrimental to mycorrhizal colonization. Nevertheless, Tausonia pullulans independently improved plant growth and yield, suggesting that this yeast may benefit tomato production without relying on mycorrhizal associations. These findings highlight the complex interactions between mycorrhizal fungi and soil yeasts in agronomic systems.
Downloads
Referências
Aguilera, P., Becerra, N., Alvear, M., Ortiz, N., Turrini, A., Azcón-Aguilar, C., ... & Borie, F. (2022). Arbuscular mycorrhizal fungi from acidic soils favors production of tomatoes and lycopene concentration. Journal of the Science of Food and Agriculture102 (6): 2352-2358. https://doi.org/10.1002/jsfa.11573
Biel, C., Camprubí, A., Lovato, P. E. & Calvet, C. (2021). On-farm reduced irrigation and fertilizer doses, and arbuscular mycorrhizal fungal inoculation improve water productivity in tomato production. Scientia Horticulturae 288: 110337. https://doi.org/10.1016/j.scienta.2021.110337
Bonfante, P. & Requena, N. (2011). Dating in the dark: how roots respond to fungal signals to establish arbuscular mycorrhizal symbiosis. Current opinion in plant biology 14 (4): 451-457. https://doi.org/10.1016/j.pbi.2011.03.014
Carvajal, M., Godoy, L., Gebauer, M., Catrileo, D. & Albornoz, F. (2024). Screening for indole-3-acetic acid synthesis and 1-aminocyclopropane-carboxylate deaminase activity in soil yeasts from Chile uncovers Solicoccozyma aeria as an effective plant growth promoter. Plant and Soil 496 (1): 83-93. https://doi.org/10.1007/s11104-023-05906-x
De Mendiburu, F. (2020). Agricolae tutorial (Version 1.3-2)—Statistical procedures for agricultural research.
Dejana L., Ramírez-Serrano, B., Rivero, J., Gamir, J., López-Ráez, J. A. & Pozo, M. J. (2022) Phosphorus availability drives mycorrhiza induced resistance in tomato. Frontiers in Plant Science 13: 1060926. https://doi.org/10.3389/fpls.2022.1060926
Fernandez, N. V., Mestre, M. C., Marchelli, P. & Fontenla, S. B. (2012). Yeast and yeast-like fungi associated with dry indehiscent fruits of Nothofagus nervosa in Patagonia, Argentina. FEMS microbiology ecology 80 (1): 179-192. https://doi.org/10.1111/j.1574- 6941.2011.01287.x. PMID:22224476
Fox, J. & Weisberg, S. (2018). An R companion to applied regression. Sage publications.
Fracchia, S., Godeas, A., Scervino, J. M., Sampedro, I., Ocampo, J. A. & Garc?a-Romera, I. (2003). Interaction between the soil yeast Rhodotorula mucilaginosa and the arbuscular mycorrhizal fungi Glomus mosseae and Gigaspora rosea. Soil Biology and Biochemistry 35 (5): 701-707. http://dx.doi.org/10.1016/S0038-0717(03)00086-5
Gallegos-Cedillo, V. M., Diánez, F., Nájera, C. & Santos, M. (2021). Plant agronomic features can predict quality and field performance: a bibliometric analysis. Agronomy 11 (11): 2305. https://doi.org/10.3390/agronomy11112305
Gollner, M. J., Püschel, D., Rydlová, J. & Vosátka, M. (2006). Effect of inoculation with soil yeasts on mycorrhizal symbiosis of maize. Pedobiologia 50 (4): 341-345. https://doi.org/10.1016/j.pedobi.2006.06.002
Hartig, F. (2020). DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R Packag version 020.
Jiang, C., Johkan, M., Hohjo, M., Tsukagoshi, S. & Maruo, T. (2017). A correlation analysis on chlorophyll content and SPAD value in tomato leaves. HortResearch 71 (71): 37-42. http://doi.org/10.20776/S18808824-71-P37
Lidoy, J., López-García, Á., Amate, C., García, J. M., Flors, V., García-Garrido, J. M.,.. & Pozo, M. J. (2023). Regulation of mycorrhizal colonization under stress in tomato depends on symbiotic efficiency. Environmental and Experimental Botany 215: 105479. https://doi.org/10.1016/j.envexpbot.2023.105479
McGonigle, T. P., Miller, M. H., Evans, D. G., Fairchild, G. L. & Swan, J. A. (1990). A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New phytologist 115 (3): 495-501. https://doi.org/10.1111/j.1469-8137.1990.tb00476.x
Mestre, M. C., Fontenla, S. & Rosa, C. A. (2014). Ecology of cultivable yeasts in pristine forests in northern Patagonia (Argentina) influenced by different environmental factors. Canadian journal of microbiology 60 (6): 371-382. https://doi.org/10.1139/cjm-2013-0897
Mestre, M. C., Fontenla, S., Bruzone, M. C., Fernández, N. V. & Dames, J. (2016). Detection of plant growth enhancing features in psychrotolerant yeasts from Patagonia (Argentina). Journal of basic microbiology 56 (10): 1-9. https://doi.org/1098-1106. 10.1002/jobm.201500728
Mestre, M. C., Severino, M. E. & Fontenla, S. (2021). Evaluation and selection of culture media for the detection of auxin-like compounds and phosphate solubilization on soil yeasts. Revista argentina de microbiología 53 (1): 1-10. https://doi.org/10.1016/j.ram.2024.12.004
Mestre, M. C., Tamayo Navarrete, M. I. & García Garrido, J. M. (2022). Exploring the yeast-mycorrhiza-plant interaction: Saccharomyces eubayanus negative effects on arbuscular mycorrhizal formation in tomato plants. Plant and Soil 479 (1): 529-542. https://doi.org/10.1007/s11104-022-05538-7
Mestre, M. C., Rosa, C. A., Safar, S. V., Libkind, D. & Fontenla, S. B. (2011). Yeast communities associated with the bulk-soil, rhizosphere and ectomycorrhizosphere of a Nothofagus pumilio forest in northwestern Patagonia, Argentina. FEMS microbiology ecology 78 (3): 531-541. https://doi.org/10.1111/j.1574-6941.2011.01183.x
Mohamed, H. M. (2015). Effect of arbuscular mycorrhizal fungus (Glomus mosseae) and soil yeasts interaction on root nodulation, N-fixation and growth of faba bean (Vichia faba). Malaysian Journal of Soil Science 19: 157-168.
Nimsi, K. A., Manjusha, K., Kathiresan, K. & Arya, H. (2023). Plant growth-promoting yeasts (PGPY), the lates t entrant for use in sustainable agriculture: a review. Journal of Applied Microbiology 134 (2): 1-11. https://doi.org/10.1093/jambio/lxac088
Phillips, J. M. & Hayman, D. S. (1970). Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British mycological Society 55 (1): 158-IN18.
Radi?, D., Karli?i?, V., ?or?evi?, J., Jovi?i?-Petrovi?, J., Kljujev, I., Lalevi?, B. & Rai?evi?, V. (2022). Soil yeasts promoting plant growth: benefits for the development of common wheat and white mustard. Zemdirbyste-Agriculture 109 (1): 27-34. https://doi.org/10.13080/z-a.2022.109.004
Sampedro, I., Aranda, E., Scervino, J. M., Fracchia, S., García-Romera, I., Ocampo, J. A. & Godeas, A. (2004). Improvement by soil yeasts of arbuscular mycorrhizal symbiosis of soybean (Glycine max) colonized by Glomus mosseae. Mycorrhiza 14: 229-234. https://doi.org/10.1007/s00572-003-0285-y
Sarabia, M., Jakobsen, I., Grønlund, M., Carreon-Abud, Y. & Larsen, J. (2018). Rhizosphere yeasts improve P uptake of a maize arbuscular mycorrhizal association. Applied Soil Ecology 125: 18-25. https://doi.org/10.1016/j.apsoil.2017.12.012
Smith, S. E. & Read, D. J. (2008). Mycorrhizal symbiosis. Academic press.
Soval-Villa, M., Wood, C. W. & Guertal, E. A. (2002). Tomato leaf chlorophyll meter readings as affected by variety, nitrogen form, and nighttime nutrient solution strength. Journal of plant nutrition 25 (10): 2129-2142. https://doi.org/10.1081/PLN-120014065
Van Der Heijden, M. G. & Horton, T. R. (2009). Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems. Journal of ecology 97 (6): 1139-1150. https://doi.org/10.1111/j.1365-2745.2009.01570.x
Vargas, M. F., Mestre, M. V., Vergara, C., Maturano, P., Petrignani, D., Pesce, V. & Vazquez, F. (2024). Residual brewer’s Saccharomyces cerevisiae yeasts as biofertilizers in horticultural seedlings: towards a sustainable industry and agriculture. Frontiers in Industrial Microbiology 2: 1360263. https://doi.org/10.3389/finmi.2024.1360263
Venegas Jaque, P., Cardozo, A., Sisón Cáceres, L. A. & Gasparetti, A. F. (2021). Elaboración de Biopreparados a partir de microorganismos del bosque para la producción frutihortícola de la Comarca Andina del paralelo 42°. http://hdl.handle.net/20.500.12123/9881
Wezel, A., Casagrande, M., Celette, F., Vian, J. F., Ferrer, A. & Peigné, J. (2014). Agroecological practices for sustainable agriculture. A review. Agronomy for sustainable development 34 (1): 1-20. ff10.1007/s13593-013-0180-7ff.
Downloads
Publicado
Como Citar
Edição
Seção
Licença
Copyright (c) 2025 Lilloa

Este trabalho está licenciado sob uma licença Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.