Lignin degradation by co-cultured fungi: current status and future perspectives
DOI:
Palabras clave:
Co-culture, Delignification, Fungi, Lignocellulose, ReviewResumen
The lignocellulosic biomass is a highly abundant and renewable resource. However, its exploitation is limited by the recalcitrance of the lignin present in the plant cell
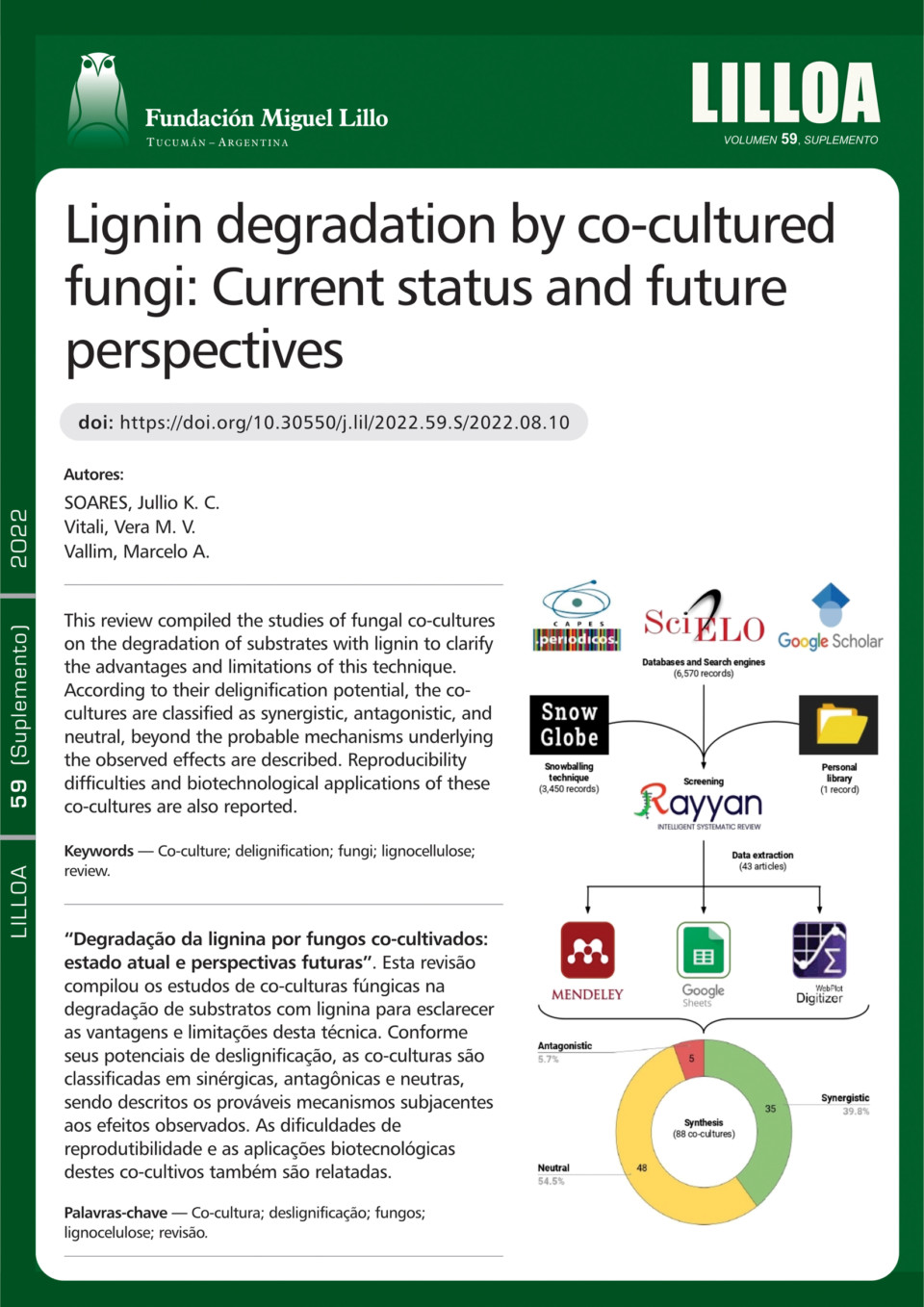
wall. In the last three decades, fungal co-cultures have increasingly been applied to overcome lignin recalcitrance by enhancing the production of ligninolytic enzymes through microbial interactions. In this paper, we systematically compile studies on fungal co-cultures used in the degradation of lignin-containing substrates to clarify the advantages and limitations of this type of culture. Based on their different delignification rate potentials, co-cultures can be classified into synergistic, antagonistic, and neutral. Co-cultivation results are generally related to the balance or imbalance of antagonistic and synergistic effects arising from the specific compatibility between the species during the interaction. It is well known that the paired species and the microenvironmental system conditions are responsible for the reported degradations,
however, the mechanisms underlying these interactions remain poorly understood. In conclusion, literature results demonstrate the promising application of fungal
co-cultures in biotechnological sectors to improve the degradation of lignin and its derivatives, through their better understanding of the efficient exploitation of
biological resources on ecological and industrial scales.
Descargas
Citas
Abdalla, M. A., Sulieman, S. & McGaw, L. J. (2017). Microbial communication: A significant approach for new leads. South African Journal of Botany 113: 461-470. doi: 10.1016/j.sajb.2017.10.001
Abdel-Hamid, A. M., Solbiati, J. O. & Caan, I. K. O. (2013). Insights into lignin degradation and its potential industrial applications. Advances in Applied Microbiology 82: 1-28. doi: 10.1016/B978-0-12-407679-2.00001-6
Adav, S. S., Ravindran, A., Cheow, E. S. H. & Sze, S. K. (2012). Quantitative proteomic analysis of secretome of microbial consortium during saw dust utilization. Journal of Proteomics 75 (18): 5590-5603. doi: 10.1016/j.jprot.2012.08.011
Albergaria, H., Francisco, D., Gori, K., Arneborg, N. & Gíria, F. (2010). Saccharomyces cerevisiae CCMI 885 secretes peptides that inhibit the growth of some non-Saccharomyces wine-related strains. Applied Microbiology and Biotechnology 86: 965-972. doi: 10.1007/s00253-009-2409-6
Albert, S. & Pandya, B. (2013). Pattern of bamboo culm degradation by Daedaleopsis confragosa when co-cultured with selected fungi. Annals of Plant Sciences 2 (12): 563-574.
Arfi, Y., Levasseur, A. & Record, E. (2013). Differential gene expression in Pycnoporus coccineus during interspecific mycelial interactions with different competitors. Applied and Environmental Microbiology 79 (21): 6626-6636. doi: 10.1128/AEM.02316-13
Arora, D. S. (1995). Biodelignification of wheat straw by different fungal associations. Biodegradation 6 (1): 57-60.
Arora, D., Gupta, P., Jaglan, S., Roullier, C., Grovel, O. & Bertrand, S. (2020). Expanding the chemical diversity through microorganisms co-culture: Current status and outlook. Biotechnology Advances 40: 1-14. doi: 10.1016/j.biotechadv.2020.107521
Arora, R., Sharma, N. K. & Kumar, S. (2018). Chapter 8 - Valorization of by-products following the biorefinery concept: commercial aspects of by-products of lignocellulosic biomass. Technologies, Commercialization, Policy Issues and Paradigm Shift for Bioethanol and By-Products 163-178. doi: 10.1016/B978-0-12-804534-3.00008-2
Asgher, M., Bhatti, H. N., Ashraf, M. & Legge, R. L. (2008). Recent developments in biodegradation of industrial pollutants by white rot fungi and their enzyme system. Biodegradation 19: 771-783. doi: 10.1007/s10532-008-9185-3
Asiegbu, F. O., Paterson, A. & Smith, J. E. (1996). The effects of co-fungal cultures and supplementation with carbohydrate adjuncts on lignin biodegradation and substrate digestibility. World Journal of Microbiology and Biotechnology 12: 273-279. doi: 10.1007/BF00360927
Asina, F., Brzonova, I., Kozliak, E., Kubátová, A. & Ji, Y. (2017). Microbial treatment of industrial lignin: Successes, problems and challenges. Renewable and Sustainable Energy Reviews 77: 1179-1205. doi: 10.1016/j.rser.2017.03.098
Bader, J., Mast-Gerlach, E., Popovic, M. K., Bajpai, R. & Stahl, U. (2010). Relevance of microbial co culture fermentations in biotechnology. Journal of Applied Microbiology 109: 371-387. doi: 10.1111/j.1365-2672.2009.04659.x
Baldrian, P. (2006). Fungal laccases – occurrence and properties. FEMS Microbiology Reviews 30 (2): 215-242. doi: 10.1111/j.1574-4976.2005.00010.x
Becker, J. & Wittmann, C. (2019). A field of dreams: Lignin valorization into chemicals, materials, fuels, and health-care products. Biotechnology Advances 37 (6): 1-24. doi: 10.1016/j.biotechadv.2019.02.016
Bergbauer, M., Moran, M. A. & Hodson, R. E. (1992). Decomposition of lignocellulose from a freshwater macrophyte by aero-aquatic fungi. Microbial Ecology 23: 159-167. doi: 10.1007/BF00172637
Bertrand, B., Martínez-Morales, F. & Trejo-Hernández, M. R. (2013). Fungal laccases: induction and production. Revista Mexicana de Ingeniería Química 12 (3): 473-488.
Bertrand, S., Bohni, N., Schnee, S., Schumpp, O., Gindro, K. & Wolfender, J. (2014). Metabolite induction via microorganism co-culture: A potential way to enhance chemical diversity for drug discovery. Biotechnology Advances 32 (6): 1180-1204. doi: 10.1016/j.biotechadv.2014.03.001
Bertrand, S., Schumpp, O., Bohni, N., Bujard, A., Azzollini, A., Monod, M., Gindro, K. & Wolfender, J. (2013a). Detection of metabolite induction in fungal co-cultures on solid media by high-throughput differential ultra-high pressure liquid chromatography–time-of-flight mass spectrometry fingerprinting. Journal of Chromatography A 1292: 219-228. doi: 10.1016/j.chroma.2013.01.098
Bertrand, S., Schumpp, O., Bohni, N., Bujard, A., Monod, M., Gindro, K. & Wolfender, J. (2013b). De Novo production of metabolites by fungal co-culture of Trichophyton rubrum and Bionectria ochroleuca. Journal of Natural Products 76 (6): 1157-1165. doi: 10.1021/np400258f
Boddy, L., Owens, E. M. & Chapela, I. H. (1989).Small scale variation in decay rate within logs one year after felling: Effect of fungal community structure and moisture content. FEMS Microbiology Ecology 5 (3): 173-183. doi: 10.1111/j.1574-6968.1989.tb03691.x
Boddy, L. (2000). Interspecific combative interactions between wood-decaying basidiomycetes. FEMS Microbiology Ecology 31 (3): 185-194. doi: 10.1111/j.1574-6941.2000.tb00683.x
Boddy, L. & Hiscox, J. (2016). Fungal ecology: Principles and mechanisms of colonization and competition by saprotrophic fungi. Microbiology Spectrum 4 (6): 1-16. doi: 10.1128/microbiolspec.FUNK-0019-2016
Carabajal, M., Levin, L., Albertó, E. & Lechner, B. (2012). Effect of co-cultivation of two Pleurotus species on lignocellulolytic enzyme production and mushroom fructification. International Biodeterioration & Biodegradation 66 (1): 71-76. doi: 10.1016/j.ibiod.2011.11.002
Carvalheiro, F., Roseiro, J. C. & Collaço, M. T. A. (1994). Biological conversion of tomato pomace by pure and mixed fungal cultures. Process Biochemistry 29 (7): 601-605. doi: 10.1016/0032-9592(94)80025-1
Chen, H. (2014). Chapter 2 Chemical composition and structure of natural lignocellulose. Biotechnology of lignocellulose: Theory and Practice. Beijing, China: Chemical Industry Press 1-47. doi: 10.1007/978-94-007-6898-7_2
Chen, Y., Huang, J., Li, Y., Zeng, G., Zhang, J., Huang, A., Zhang, J., Ma, S., Tan, X., Xu, W. & Zhou, W. (2015). Study of the rice straw biodegradation in mixed culture of Trichoderma viride and Aspergillus niger by GC-MS and FTIR. Environmental Science and Pollution Research 22: 9807-9815. doi: 10.1007/s11356-015-4149-8
Chi, Y., Hatakka, A. & Maijala, P. (2007). Can co-culturing of two white-rot fungi increase lignin degradation and the production of lignin-degrading enzymes?. International Biodeterioration & Biodegradation 59 (1): 32-39. doi: 10.1016/j.ibiod.2006.06.025
Chio, C., Sain, M. & Qin, W. (2019). Lignin utilization: A review of lignin depolymerization from various aspects. Renewable and Sustainable Energy Reviews 107: 232-249. doi: 10.1016/j.rser.2019.03.008
Cui, T., Yuan, B., Guo, H., Tian, H., Wang, W., Ma, Y., Li, C. & Fei, Q. (2021). Enhanced lignin biodegradation by consortium of white rot fungi: microbial synergistic effects and product mapping. Biotechnology for Biofuels 14 (162): 1-11. doi: 10.1186/s13068-021-02011-y
Cui, Y., Dong, X., Tong, J. & Liu, S. (2015). Degradation of lignocellulosic components in un-pretreated vinegar residue using an artificially constructed fungal consortium. BioResources 10 (2): 3434-3450. doi: 10.15376/biores.10.2.3434-3450
Darwish, G. A. M. A., Bakr, A. A. & Abdallah, M. M. F. (2012). Nutritional value upgrading of maize stalk by using Pleurotus ostreatus and Saccharomyces cerevisiae in solid state fermentation. Annals of Agricultural Sciences 57 (1): 47-51. doi: 10.1016/j.aoas.2012.03.005
Dashtban, M., Schraft, H., Syed, T. A. & Qin, W. (2010). Fungal biodegradation and enzymatic modification of lignin. International Journal of Biochemistry and Molecular Biology 1 (1): 36-50.
Dong, X., Dong, M., Lu, Y., Turley, A., Jin, T. & Wu, C. (2011). Antimicrobial and antioxidant activities of lignin from residue of corn stover to ethanol production. Industrial Crops and Products 34 (3): 1629-1634. doi: 10.1016/j.indcrop.2011.06.002
Dullah, S., Hazarika, D. J., Parveen, A., Kakoti, M., Borgohain, T., Gautom, T., Bhattacharyya, A., Barooah, M. & Boro, R. C. (2021). Fungal interactions induce changes in hyphal morphology and enzyme production. Mycology: An International Journal on Fungal Biology 12 (1): 279-295. doi: 10.1080/21501203.2021.1932627
Evans, J. A., Eyre, C. A., Rogers, H. J., Boddy, L. & Muller, C. T. (2008). Changes in volatile production during interspecific interactions between four wood rotting fungi growing in artificial media. Fungal Ecology 1 (2-3): 57-68. doi: 10.1016/j.funeco.2008.06.001
Ezeonu, C. S., Onwurah, I. N. E., Ubani, C. S., Ejikeme, C. M. & Ogodo, A. C. (2016). Trichophyton soudanense and Trichophyton mentagrophyte - treated rice husk biomass components and effect of yeast on the bioethanol yield. Achievements in the Life Sciences 10 (1): 72-79. doi: 10.1016/j.als.2016.05.007
Fakruddin, M., Hossain, M. N. & Ahmed, M. M. (2017). Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complementary and Alternative Medicine 17 (64): 1-11. doi: 10.1186/s12906-017-1591-9
Fanelli, D. (2010). Do pressures to publish increase scientists' bias? An empirical support from US states data. PLOS ONE 5 (4): 1-7. doi: 10.1371/journal.pone.0010271
Fatma, S., Saleem, A. & Tabassum, R. (2021). Wheat straw hydrolysis by using co-cultures of Trichoderma reesei and Monascus purpureus toward enhanced biodegradation of the lignocellulosic biomass in bioethanol biorefinery. Biomass Conversion and Biorefinery 11: 743-754. doi: 10.1007/s13399-020-00652-x
Feng, N., Ma, Q., Yuan, M., Zhai, H. & Ek, M. (2015). Improving degradation ability toward wheat straw chemical composition by co-cultivation of Pycnoporus sanguineus with Candida tropicalis. Journal of Biobased Materials and Bioenergy 9 (6): 567-571. doi: 10.1166/jbmb.2015.1555
Feng, N., Zhai, H. & Lai, Y. (2016). On the chemical aspects of the biodelignification of wheat straw with Pycnoporus sanguineus and its combined effects with the presence of Candida tropicalis. Industrial Crops and Products 91: 315-322. doi: 10.1016/j.indcrop.2016.07.035
Flores, C., Casasanero, R., Trejo-Hernández, M. R., Galindo, E. & Serrano-Carreón, L. (2010). Production of laccases by Pleurotus ostreatus in submerged fermentation in co-culture with Trichoderma viride. Journal of Applied Microbiology 108 (3): 810-817. doi: 10.1111/j.1365-2672.2009.04493.x
Gamal, R. F., Abdelhady, H. M., Nageeb, Z. A. & Elgarhy, E. A. (2014). Pulping of sugarcane bagasse using Ceriporiopsis subvermispora SS-33 and Ophiostoma piliferum as a fungal bio-agents. Egyptian Journal of Microbiology 49 (1): 1-15. doi: 10.21608/EJM.2014.239
García, A., Spigno, G. & Labidi, J. (2017). Antioxidant and biocide behavior of lignin fractions from apple tree pruning residues. Industrial Crops and Products 104: 242-252. doi: 10.1016/j.indcrop.2017.04.063
Giles, R. L., Galloway, E. R., Zackeru, J. C., Naithani, V. & Parrow, M. W. (2014). Two stage fungal biopulping solubilizes lignocellulosic carbohydrates without supplemental enzymatic hydrolysis. International Biodeterioration & Biodegradation 86: 265-271. doi: 10.1016/j.ibiod.2013.09.016
Giles, R. L., Zackeru, J. C., Galloway, E. R., Elliott, G. D. & Parrow, M. W. (2015). Single versus simultaneous species treatment of wood with Ceriporiopsis subvermispora and Postia placenta for ethanol applications, with observations on interspecific growth inhibition. International Biodeterioration & Biodegradation 99: 66-72. doi: 10.1016/j.ibiod.2014.11.005
Goers, L., Freemont, P. & Polizzi, M. (2014). Co-culture systems and technologies: taking synthetic biology to the next level. Journal of the Royal Society Interface 11 (96): 1-13. doi: 10.1098/rsif.2014.0065
Gu, H., Zhu, Y., Peng, Y., Liang, X., Liu, X., Shao, L., Xu, Y., Xu, Z., Liu, R. & Li, J. (2019). Physiological mechanism of improved tolerance of Saccharomyces cerevisiae to lignin-derived phenolic acids in lignocellulosic ethanol fermentation by short-term adaptation. Biotechnology for Biofuels 12 (268): 1-14. doi: 10.1186/s13068-019-1610-9
Guerriero, G., Hausman, J., Strauss, J., Ertan, H. & Siddiqui, K. S. (2016). Lignocellulosic biomass: Biosynthesis, degradation, and industrial utilization. Engineering in Life Sciences 16: 1-16. doi: 10.1002/elsc.201400196
Hermosilla, E., Rubilar, O., Schalchli, H., Silva, A. S., Ferreira-Leitao, V. & Cristina Diez, M. (2018). Sequential white-rot and brown-rot fungal pretreatment of wheat straw as a promising alternative for complementary mild treatments. Waste Management 79: 240-250. doi: 10.1016/j.wasman.2018.07.044
Hiscox, J. & Boddy, L. (2017). Armed and dangerous - Chemical warfare in wood decay communities. Fungal Biology Reviews 31 (4): 169-184. doi: 10.1016/j.fbr.2017.07.001
Hiscox, J., O’leary, J. & Boddy, L. (2018). Fungus wars: basidiomycete battles in wood decay. Studies in Mycology 89: 117-124. doi: 10.1016/j.simyco.2018.02.003
Holmer, L. & Stenlid, J. (1993). The importance of inoculum size for the competitive ability of wood decomposing fungi. FEMS Microbiology Ecology 12 (3): 169-176. doi: 10.1111/j.1574-6941.1993.tb00029.x
Horsley, T., Dingwall, O. & Sampson, M. (2011). Checking reference lists to find additional studies for systematic reviews. Cochrane Database of Systematic Reviews 8: 1-27. doi: 10.1002/14651858.MR000026.pub2
Huisman, J. & Weissing, F. J. (2001). Fundamental unpredictability in multispecies competition. The American Naturalist 157 (5): 488-494. doi: 10.1086/319929
Iakovlev, A. & Stenlid, J. (2000). Spatiotemporal patterns of laccase activity in interacting mycelia of wood-decaying basidiomycete fungi. Microbial Ecology 39 (3): 236-245. doi: 10.1007/s002480000022
Ijoma, G. N. & Tekere, M. (2017). Potential microbial applications of co-cultures involving ligninolytic fungi in the bioremediation of recalcitrant xenobiotic compounds. International Journal of Environmental Science and Technology 14: 1787-1806. doi: 10.1007/s13762-017-1269-3
Jamal, P., Saheed, O. K., Karim, M. I. A., Alam, M. Z. & Muyibi, S. A. (2015). A fermentative approach to ameliorating solid waste challenges within food and hospitality industry. International Biodeterioration & Biodegradation 102: 182-190. doi: 10.1016/j.ibiod.2015.03.031
Jiang, L., Zhou, J., Quan, C. & Xiu, Z. (2017). Advances in industrial microbiome based on microbial consortium for biorefinery. Bioresour Bioprocess 4 (11): 1-10. doi: 10.1186/s40643-017-0141-0
Karpe, A. V., Beale, D. J., Harding, I. H. & Palombo, E. A. (2014). Optimization of degradation of winery-derived biomass waste by Ascomycetes. Chemical Technology and Biotechnology 90 (10): 1793-1801. doi: 10.1002/jctb.4486
Kaur, P., Kocher, G. S. & Taggar, M. S. (2018). Development of fungal consortium for the pretreatment of rice straw under optimized solid state and shake flask conditions. Environmental Progress & Sustainable Energy 38 (2): 635-646. doi: 10.1002/ep.12954
Ke, L., Wu, Q. & Zhang, D. (2011). Bioconversion of rape straw into a nutritionally enriched substrate by Ganoderma lucidum and yeast. African Journal of Biotechnology 10 (29): 5648-5653.
Koroleva, O. V., Gavrilov, V. P., Stepanova, E. V., Lebedeva, V. I., Sverdlova, N. I., Landesman, E. O., Yavmetdinov, I. S. & Yaropolov, A. I. (2002). Production of lignin modifying enzymes by co-cultivated white-rot fungi Cerrena maxima and Coriolus hirsutus and characterization of laccase from Cerrena maxima. Enzyme and Microbial Technology 30 (4): 573-580. doi: 10.1016/S0141-0229(02)00021-2
Košíková, B. & Sláviková, E. (1996). Growth of Saccharomyces cerevisiae, Rhodotorula rubra and Bullera alba in the presence of beechwood prehydrolyzate-based lignin fractions. Folia Microbiologica 41: 430–432. doi: 10.1007/BF02815694
Kuhar, F., Castiglia, V. & Levin, L. (2015). Enhancement of laccase production and malachite green decolorization by co-culturing Ganoderma lucidum and Trametes versicolor in solid-state fermentation. International Biodeterioration & Biodegradation 104: 238-243. doi: 10.1016/j.ibiod.2015.06.017
Kumar, A., Gautam, A. & Dutt, D. (2020). Bio-pulping: An energy saving and environment-friendly approach. Physical Sciences Reviews 5 (10): 1-9. doi: 10.1515/psr-2019-0043
Larroy, C., Fernández, M. R., González, E., Parés, X. & Biosca, J. A. (2003). Properties and functional significance of Saccharomyces cerevisiae ADHVI. Chemico-Biological Interactions 143-144: 229-238. doi: 10.1016/s0009-2797(02)00166-7
Li, M., Wang, Z., Sun, J., Chen, W., Hou, X. & Gao, Z. (2021). Synergistic effect of mixed fungal pretreatment on thermogravimetric characteristics of rice straw. BioResources 16 (2): 3978-3990. doi: 10.15376/biores.16.2.3978-3990
Lundell, T. K., Mäkelä, M. R., Vries, R. P. & Hildén, K. S. (2014). Chapter 11 – Genomics, lifestyles and future prospects of wood-decay and litter-decomposing Basidiomycota. Advances in Botanical Research 70: 329-370. doi: 10.1016/B978-0-12-397940-7.00011-2
Luo, R., Liao, Q., Xia, A., Deng, Z., Huang, Y., Zhu, X. & Zhu, X. (2020). Synergistic treatment of alkali lignin via fungal coculture for biofuel production: Comparison of physicochemical properties and adsorption of enzymes used as catalysts. Frontiers in Energy Research 8 (575371): 1-10. doi: 10.3389/fenrg.2020.575371
Ma, F., Wang, J., Zeng, Y., Yu, H., Yang, Y. & Zhang, X. (2011). Influence of the co-fungal treatment with two white rot fungi on the lignocellulosic degradation and thermogravimetry of corn stover. Process Biochemistry 46 (9): 1767-1773. doi: 10.1016/j.procbio.2011.05.020
Meehnian, H., Jana, A. K. & Jana, M. M. (2017). Pretreatment of cotton stalks by synergistic interaction of Daedalea flavida and Phlebia radiata in co-culture for improvement in delignification and saccharification. International Biodeterioration & Biodegradation 117: 68-77. doi: 10.1016/j.ibiod.2016.11.022
Nazarpour, F., Abdullah, D. K., Abdullah, N. & Zamiri, A. (2013). Evaluation of biological pretreatment of rubberwood with white rot fungi for enzymatic hydrolysis. Materials 6 (5): 2059-2073. doi: 10.3390/ma6052059
Owens, E. M., Reddy, C. A. & Grethlein, H. E. (1994). Outcome of interspecific interactions among brown-rot and white-rot wood decay fungi. FEMS Microbiology Ecology 14 (1): 19-24. doi: 10.1111/j.1574-6941.1994.tb00086.x
Qadir, F., Shariq, M., Ahmed, A. & Sohail, M. (2018). Evaluation of a yeast co-culture for cellulase and xylanase production under solid state fermentation of sugarcane bagasse using multivariate approach. Industrial Crops and Products 123: 407-415. doi: 10.1016/j.indcrop.2018.07.021
Ramamurthy, V., Cheepurupalli, L., Rathore, S. S. & Ramakrishnan, J. (2017). Co-culture: A promising method in enzyme production. International Journal of ChemTech Research 10 (6): 720-726.
Rayner, A. D. M. & Boddy, L. (1988). Fungal communities in the decay of wood. Advances in Microbial Ecology 10: 115-166.
Sasongko, W. T., Larasati, T. R. D., Mulyana, N. & Wahyono, T. (2019). In vitro gas and methane production from fermented rice straw using Trichoderma viride and Phanerochaete chrysosporium inoculant. Materials Science and Engineering 546: 1-7. doi: 10.1088/1757-899X/546/2/022023
Savoie, J. M., Mata, G. & Billette, C. (1998). Extracellular laccase production during hyphal interactions between Trichoderma sp. and shiitake, Lentinula edodes. Applied Microbiology and Biotechnology 49: 589–593. doi: 10.1007/s002530051218
Score, A. J., Palfreyman, J. W. & White, N. A. (1997). Extracellular phenoloxidase and peroxidase enzyme production during interspecific fungal interactions. International Biodeterioration & Biodegradation 39 (2-3): 225-233. doi: 10.1016/S0964-8305(97)00012-7
Sharma, R. K. & Arora, D. S. (2013). Fungal degradation of lignocellulosic residues: An aspect of improved nutritive quality. Critical Reviews in Microbiology 41 (1): 52-60. doi: 10.3109/1040841X.2013.791247
Silva, J. A. T. (2015). Negative results: negative perceptions limit their potential for increasing reproducibility. Journal of Negative Results in BioMedicine 14 (12): 1-4. doi: 10.1186/s12952-015-0033-9
Singh, A. P. & Singh, T. (2014). Biotechnological applications of wood-rotting fungi: A review. Biomass and Bioenergy 62: 198-206. doi: 10.1016/j.biombioe.2013.12.013
Singh, P., Sulaiman, O., Hashim, R., Rupani, P. F. & Peng, L. C. (2010). Biopulping of lignocellulosic material using different fungal species: a review. Reviews in Environmental Science and Bio/Technology 9: 141-151. doi: 10.1007/s11157-010-9200-0
Song, Z., Vail, A., Sadowsky, M. J. & Schilling, J. S. (2012). Competition between two wood-degrading fungi with distinct influences on residues. FEMS Microbiology Ecology 79 (1): 109-117. doi: 10.1111/j.1574-6941.2011.01201.x
Sperandio, G. B. & Filho, E. X. F. (2019). Fungal co-cultures in the lignocellulosic biorefinery context: A review. International Biodeterioration & Biodegradation 142: 109-123. doi: 10.1016/j.ibiod.2019.05.014
Stepanova, E. V., Koroleva, O. V., Vasilchenko, L. G., Karapetyan, K. N., Landesman, E. O., Yavmetdinov, I. S., Kozlov, Y. P. & Rabinovich, M. L. (2003). Fungal decomposition of oat straw during liquid and solid-state fermentation. Applied Biochemistry and Microbiology 39: 65-74. doi: 10.1023/A:1021702211169
Sundman, V. & Näse, L. (1972). The synergistic ability of some wood-degrading fungi to transform lignins and lignosulfonates on various media. Archiv für Mikrobiologie 86: 339-348. doi: 10.1007/bf00424990
Tao, L., Zhang, L. X., Tu, Y., Zhang, N. F., Si, B. W., Ma, T. & Diao, Q. Y. (2016). Improving the in situ ruminal degradability of maize stalk using fungal inoculants in dorper × thin-tailed han crossbred ewes. Small Ruminant Research 144: 119-125. doi: 10.1016/j.smallrumres.2016.09.001
Tsujiyama, S. & Minami, M. (2005). Production of phenol-oxidizing enzymes in the interaction between white-rot fungi. Mycoscience 43 (4): 268-271. doi: 10.1007/S10267-005-0243-Y
Ujor, V. C., Adukwu, E. C. & Okonkwo, C. C. (2018). Fungal wars: The underlying molecular repertoires of combating mycelia. Fungal Biology 122 (4): 191-202. doi: 10.1016/j.funbio.2018.01.001
Van Dyk, J. S. & Pletschke, B. I. (2012). A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes - Factors affecting enzymes, conversion and synergy. Biotechnology Advances 30 (6): 1458-1480. doi: 10.1016/j.biotechadv.2012.03.002
Van Heerden, A., Roux, N. J., Swart, J., Lubbe-Gardner, S. & Botha, A. (2008). Assessment of wood degradation by Pycnoporus sanguineus when co-cultured with selected fungi. World Journal of Microbiology and Biotechnology 24 (11): 2489-2497. doi: 10.1007/s11274-008-9773-8
Vanholme, R., Demedts, B., Morreel, K., Ralph, J. & Boerjan, W. (2010). Lignin biosynthesis and structure. Plant Physiology 153: 895-905. doi: 10.1104/pp.110.155119
Vasil'chenko, L. G., Karapetyan, K. N., Yachkova, S. N., Zernova, E. S. & Rabinovich, M. L. (2004). Degradation of a lignin–carbohydrate substrate by soil fungi producing laccase and cellobiose dehydrogenase. Applied Biochemistry and Microbiology 40: 44-49. doi: 10.1023/b:abim.0000010350.17045.1c
Wang, H., Peng, L., Ding, Z., Wu, J. & Shi, G. (2015). Stimulated laccase production of Pleurotus ferulae JM301 fungus by Rhodotorula mucilaginosa yeast in co-culture. Process Biochemistry 50 (6): 901-905. doi: 10.1016/j.procbio.2015.03.004
Wang, W., Yuan, T. & Cui, B. (2014). Biological pretreatment with white rot fungi and their co-culture to overcome lignocellulosic recalcitrance for improved enzymatic digestion. BioResources 9 (3): 3968-3976.
White, N. A. & Boddy, L. (1992). Extracellular enzyme localization during interspecific fungal interactions. FEMS Microbiology Letters 98 (1-3): 75-80. doi: 10.1111/j.1574-6968.1992.tb05493.x
Woodward, S. & Boddy, L. (2008). Chapter 7 - Interactions between saprotrophic fungi. British Mycological Society Symposia Series 28: 125-141. doi: 10.1016/S0275-0287(08)80009-4
Xie, P., Fan, L., Huang, L. & Zhang, C. (2020). An innovative co-fungal treatment to poplar bark sawdust for delignification and polyphenol enrichment. Industrial Crops and Products 157: 1-11. doi: 10.1016/j.indcrop.2020.112896
Yang, D., Billerbeck, G. M., Zhang, J., Rosenzweig, F. & Francois, J. (2017). Deciphering the origin, evolution, and physiological function of the subtelomeric aryl-alcohol dehydrogenase gene family in the yeast Saccharomyces cerevisiae. Applied and Environmental Microbiology 84 (1): 1-16. doi: 10.1128/AEM.01553-17
Yang, R., Meng, D., Hu, X., Ni, Y. & Li, Q. (2013). Saccharification of pumpkin residues by coculturing of Trichoderma reesei rut-c30 and Phanerochaete chrysosporium burdsall with delayed inoculation timing. Journal of Agricultural and Food Chemistry 61 (38): 9192-9199. doi: 10.1021/jf402199j
Yang, Y. S., Zhou, J. T., Lu, H., Yuan, Y. L. & Zhao, L. H. (2011). Isolation and characterization of a fungus Aspergillus sp. strain F-3 capable of degrading alkali lignin. Biodegradation 22: 1017-1027. doi: 10.1007/s10532-011-9460-6
Yao, L., Zhu, L., Xu, X., Tan, L., Sadilek, M., Fan, H., Shen, X., Yang, J., Qiao, B. & Yang, S. (2016). Discovery of novel xylosides in co-culture of basidiomycetes Trametes versicolor and Ganoderma applanatum by integrated metabolomics and bioinformatics. Scientific Reports 6: 332-337. doi: 10.1038/srep33237
Yavmetdinov, I. S., Stepanova, E. V., Gavrilova, V. P., Lokshin, B. V., Perminova, I. V. & Koroleva, O. V. (2003). Isolation and characterization of humin-like substances produced by wood-degrading white rot fungi. Applied Biochemistry and Microbiology 39: 257-264. doi: 10.1023/A:1023571426331
Zhang, J., Ke, W. & Chen, H. (2019). Enhancing laccase production by white-rot fungus Trametes hirsuta SSM-3 in co-culture with yeast Sporidiobolus pararoseus SSM-8. Preparative Biochemistry & Biotechnology 50 (1): 1-8. doi. 10.1080/10826068.2019.1655764
Zhong, Z., Li, N., He, B., Igarashi, Y. & Luo, F. (2019). Transcriptome analysis of differential gene expression in Dichomitus squalens during interspecific mycelial interactions and the potential link with laccase induction. Journal of Microbiology 57 (2): 127-137. doi: 10.1007/s12275-019-8398-y
Zhou, L. & Li, L. Y. (2016). Novel fungal consortium pretreatment of waste oat straw to enhance economical and efficient biohydrogen production. Ecocycles 2 (2): 36-42. doi: 10.19040/ecocycles.v2i2.61
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2022 Lilloa

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.